Abstract
Background: ROR1 is a cell surface receptor that is largely absent or expressed at low levels in healthy adult tissues but is expressed at high levels on a wide range of hematological malignancies as well as solid tumours, making it an ideal anti-cancer drug target. NVG-111 is a bispecific T cell engager (TCE) antibody targeting ROR1xCD3 using a tandem scFv format to redirect the cytotoxic activity of T cells to ROR1+ tumour cells. NVG-111 is currently showing efficacy with predictable and manageable toxicity in Phase I/II clinical development for the treatment of Chronic Lymphocytic Leukaemia (CLL) and Mantle Cell Lymphoma (MCL), with the scope to expand into other hematological malignancies and solid tumour indications. These clinical data provide the foundation for the clinical development of a half-life extended (HLE) TCE using the clinically derisked ROR1 and CD3 binders from NVG-111.
Objective: Building on the safety and efficacy data emerging from the Phase I/II trial of NVG-111, develop a half-life extended ROR1xCD3 TCE for a more convenient once a week dosing regimen, without compromising the safety, efficacy and CMC attributes of NVG-111.
Methods: Utilizing NVG-111 as a core for a ROR1xCD3 HLE-TCE, several formats were designed and evaluated. These incorporated different half-life extending technology solutions by increasing the molecule size and enabling FcRn-mediated recycling. NVG-111 was cloned in-format with an antibody Fc, human serum albumin (HSA), or an HSA-binding antibody into a suitable expression vector and the resultant clones expressed using Expi293 cells. Molecules were then purified using our proprietary downstream purification process. The potency of ROR1 targeting HLE-TCEs were evaluated in cytotoxicity and T cell activation co-culture assays using MCL derived JeKo-1 target cells and T cells from healthy donor PBMCs, with responses measured by flow cytometry. Biophysical properties were evaluated by determining the post-purification expression titer, aggregation profile, and stability at 37°C.
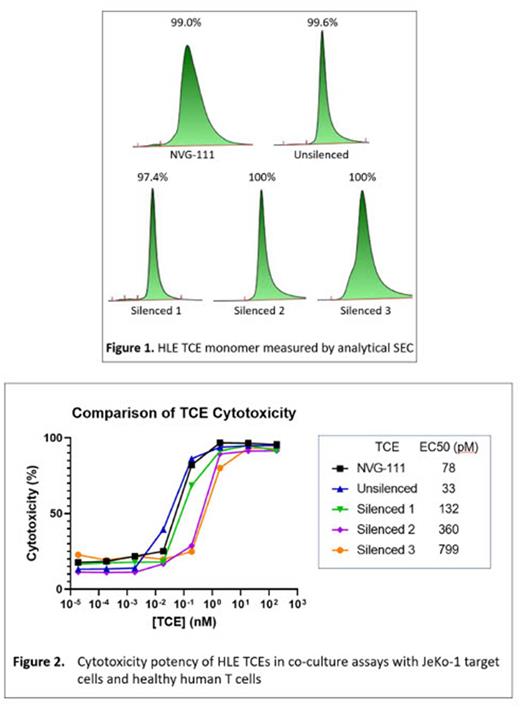
Results: Reformatting NVG-111 into an IgG-like bispecific scFv-Fc heterodimer resulted in loss of cytotoxic potency, which may be due to a change in the geometry of binding or poorer target engagement. Fusing NVG-111 directly to an HSA-binding antibody resulted in complete loss of expression. A 3-fold reduction in expression titer was observed for NVG-111 fused directly with HSA, which was also accompanied with a >10-fold reduction in functional activity, loss of binding affinity and reduced stability compared with parental molecule. Fusing NVG-111 to Fc resulted in a molecule that maintained functional potency, with transient expression titer remaining >100mg/L, which is also aligned with the parental molecule. The lead Fc-fused HLE molecule was then further refined with appropriate Fc-silencing technologies, which displayed low levels of aggregation following purification, with a monomer content of >97% (Figure 1). Stability studies performed in formulation buffer and in human serum showed that the Fc-fused HLE molecule was at least as stable as parental NVG-111, retaining binding and no evidence of aggregation or fragmentation. The potency of HLE-TCE with respect to cytotoxicity was influenced by the silencing technologies (Figure 2), with technologies 1 and 2 retaining potency as measured by EC50 to within 2-fold to 5-fold of NVG-111. However, silencing technology 3 displayed a 10-fold lower potency than NVG-111 and was therefore not progressed. Finally, initial PK studies suggest that our selected ROR1 targeting HLE-TCE has a half-life that would support weekly dosing in humans.
Conclusion: We have generated a first in class ROR1 targeting half-life extended TCE that maintains the functional and biophysical properties of the clinically promising NVG-111. Our systematic evaluation suggests that careful selection of HLE format is critical for the development of a molecule with good yield, biophysical characteristics and potency. The selected ROR1 HLE-TCE supports weekly administration whilst maintaining potency at sub-nanomolar concentrations, properties which differentiate it from other TCEs in development. IND enabling studies with our 1st in class ROR1 targeting HLE-TCE will conclude in 2023, supporting the initiation of a Phase I clinical trial in ROR1+ hematological malignancies.
Disclosures
Granger:Haleon: Current equity holder in publicly-traded company; GSK: Current equity holder in publicly-traded company, Patents & Royalties. Shah:UCB: Divested equity in a private or publicly-traded company in the past 24 months, Ended employment in the past 24 months. Chester:Informa PLC: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Patents & Royalties; Allen & Overy LLP: Consultancy; UCL Business: Patents & Royalties; Novalgen: Consultancy, Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties. Nathwani:Biomarin: Research Funding; Genethon: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal